Explain How Monomers and Polymers Are Different From Each Other
Macromolecules in biology refers to the major categories of molecules that make the cell. An addition polymer is made of only one kind of monomer.

Monomers And Polymers Role Importance Expii
Small molecule that forms covalent bonds with other small molecules to produce a large molecule called a polymer.
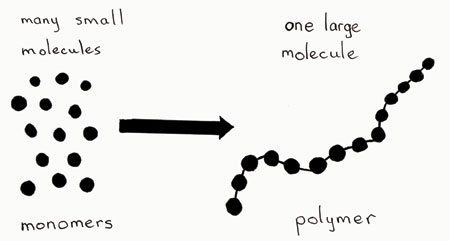
. These monomers are linked to each other via covalent bonds through a process called polymerization. Difference Between Monomer and Polymer Definition. The small molecular units are called monomers mono means one or single and they are linked together into long chains called polymers poly means many or multiple.
An analogy is a chain. Is the way cells break down different polymers the same for each polymer. For example polythene is a homopolymer of ethane.
An addition polymer is made when monomers bond to each other without the loss of atoms. A condensation polymer is. A condensation polymer is made of only one kind of monomer.
Macromolecules are big molecules macro means big opposite to micro which is small. An addition polymer is made of only one kind of monomer. Each monomer is a molecule which is chemically identical to the preceding and following monomer in the chain which is one larger molecule.
Hydrolysis is one way where water is used to disassemble polymers. -Nucleotides form nucleic acids eg. Each different type of macromolecule except lipids is built from a different set of monomers that resemble each other in composition and size.
An addition polymer is made when monomers bond to each other without the loss of atoms. A monomer is a single repeating unit that is covalently bound to form polymers. A monomer is the single unit of a polymer - polymer means many monomers.
A polymer is the chain and monomers are the links in the chain. Monomers are simple molecules with low. Dyhydration synthesis is the opposite where a polymer is built by the subtraction of water explain the relationship between monomers and polymers and explain how these molecules are related to.
A condensation polymer consists of two different kinds of monomers. You have two components which are organic polymers of different molecular weights and with different composition of each other one of them can be a co-polymer made with two different monomers covalently linked in the chain the other is a homopolymer with a chain formed by. So withpolysaccharides being polymers or monomers linked together then think of a single monomer of sugar such as maltose.
-Fatty acids are the monomers for lipids for example and regardless of how they are bonded as a saturated or unsaturated fat for example they will form lipids. A condensation polymer is. How monomers are connected.
Monomers are single units while polymers are monomers linked together. An addition polymer consists of two different kinds of monomers. Monomers are small molecules mostly organic that can join with other similar molecules to form polymers.
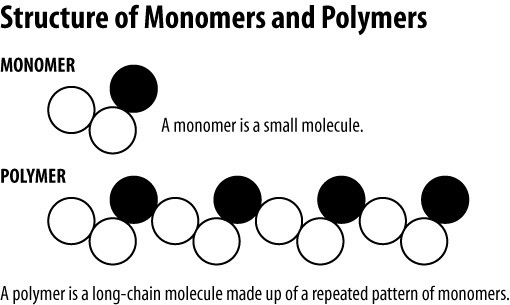
The polymers that are formed by the polymerization of a single monomer are known as a homopolymer. Ie a polymer is many chemically bound repeating monomers. A condensation polymer consists of two different kinds of monomers.
The polymers whose repeating units are derived from two types of monomers are known as copolymers. Each unit can be combined to make a many unit molecule or polymer. Polymers are complex molecules with very high molecular weight.
Large molecule that consists of many smaller molecules called monomers joined together by covalent bonds. In other words the repeating units of homopolymers are derived only from one monomer. Monomers are the building block of polymers.
Monomer is a word made of two parts mono means one and mer means unit so monomers are the building units of the polymersPoly means many. While there is variation among the types of biological polymers found in different organisms the chemical mechanisms for assembling and disassembling them are largely the same across organisms. Each unit is called a monomer or single while polymers are many.
Polysaccharides are often quite heterogeneous. In the process of polymerization monomers are linked to each other forming a polymer chain. Explain how you would separate all the components of Question.
Monomers are smaller molecules and when bonded together make up polymers. They have high molecular weight high boiling point higher mechanical strength higher complexity and lesser chemical reactivity. Polymers are comprised of a chain of monomers through a process known as polymerization.
A condensation polymer is made of only one kind of monomer. These monomers can be of one type or more than one type. An addition polymer consists of two different kinds of monomers.
Process by which 2 monomers are covalently bonded to each other with the loss of a water molecule one. Polymers are basically repeats of monomers. They can exist as either linear simple structures or branched complex structures.
These polymers have very high masses and densities. Polymers are the macromolecules formed by the repeating units of monomers. The main difference between monomers and polymers is that the former is the necessary component that forms the latter.
Monomers are generally linked together through a process called dehydration synthesis while polymers are disassembled through a process called hydrolysis. A polymer is a macroscopic material built from a large number of repeating single units bound together. Monomers have high chemical reactivity and they react with the same or different monomer molecules to form a large structure called a polymer.
The _____ of a large biological molecules helps explain how it works.
What Are The Monomers And Polymers Of Protein Socratic
Difference Between Monomer And Polymer

What Is The Difference Between Monomers And Polymers Socratic
No comments for "Explain How Monomers and Polymers Are Different From Each Other"
Post a Comment